

|
Senior Design 2008—2009, Group 2 |
|
National High Magnetic Field Laboratory (NHMFL) 900 MHz NMR Probe |
|
Background Information |
|
Nuclear Magnetic Resonance Nuclear magnetic resonance (NMR) spectroscopy allows scientists to assess chemical and structural properties of a sample in non-destructive manner. The technique was first applied to liquid and solid samples in 1946 by Felix Bloch and Edward Mills Purcell. NMR spectroscopy of liquid samples has since matured as a scientific discipline and for the last several decades following this discovery has been an important tool in the pharmaceutical and chemical industries. Application of NMR to solid-state samples followed as techniques such as magic-angle spinning were developed to increase their inherently poor signal resolution. NMR takes advantage of a quantum property of all atomic particles called spin. Electrons, neutrons, and protons each exhibit this property, with individual spin values of 1/2. NMR spectroscopy is measured from the interactions of applied radio frequency (RF) electromagnetic radiation to the spin polarity of neutrons and protons – hence the name nuclear magnetic resonance. Only isotopes which have unpaired neutron and or proton spins are directly observable using NMR. Isotope examples include 1H, 2H, 15N, 31P, 14N, 13C, and 19F. When a sample containing NMR-sensitive isotopes is placed in an external magnetic field, 900 MHz Magnet called the B0 field, some spins align themselves in a low energy state opposite to B0 magnetic polarity, while the remainder align in a high energy state with the same B0 magnetic polarity. Photons of frequency v cause a spin energy level jump, where the frequency is proportional to the B0 field multiplied by a proportionality constant specific to the isotope, called the gyromagnetic ratio, γ.
On a macroscopic scale, the average of all the individual spin polarities yields the net magnetization, M0. As RF energy is applied at the resonant frequency of the isotopes being studied, the net magnetization vector along the Z axis can be brought to zero. Additionally, if enough energy is applied, the net magnetization vector can be brought into the XY plane, where it will rotate around the Z axis at the v frequency of the photons which cause the energy level jumps. This is called the frequency of precession or the Larmor frequency. After the net magnetization is brought into precession in the XY plane, scientists measure the time it takes for it to return to zero along the Z axis. Also, the time for the vector to return to equilibrium along the Z axis at M0 is measured. Both of these measurements are respectively called the T2 and T1 relaxation times. Through Fourier transforms, these relaxation response times are converted to the frequency domain. In this way the frequency peaks in an unknown sample can be compared to known frequencies of the elements, and the atomic ordering of a molecule can be determined. In addition, other more complex NMR techniques allow the inter-atomic distances and angles to be determined within a molecule, so that the 3-D structure of the molecule can be known.
For modern NMR experiments, a solid or liquid sample is placed inside a probe which locates the sample in the center of the B0 magnetic field created by a superconducting electromagnet. The weak signal to noise ratio of NMR spectroscopy requires signal averaging over time, so a homogeneous magnetic field is needed across the sample so that field variations do not overly skew the frequency peaks. In order to increase field homogeneity, non-superconducting shim coils are placed around the probe bore and currents are applied for various terms, such as Z, Z2, X, Y, XY, X2, and so on. In the NMR probe, the sample is place in a solenoid coil oriented in the X direction. When RF pulses are applied to this coil, a B1 field is created which acts to bring the net magnetization vector into precession in the XY plane. The simple NMR experiment consists of pulses to bring the net spin vector into precession, followed by an acquisition phase, where the frequency of the rotating magnetization vector induces current in the solenoid which is amplified and recorded by the spectrometer console.
Magic Angle Spinning For non-crystalline chemical samples in the solid-state, dipolar and quadripolar interactions between atoms and also chemical shift anisotropy cause the spectral lines to become broad. Line broadening serves to mask individual frequency peaks, and so information about the atomic structure of a molecule is lost. By spinning the sample at 54.74° to the B0 vector, the frequency peaks can be narrowed, raising the resolution. This technique is referred to as magic angle spinning (MAS), where spin rates of 1 to 70 kHz are applied to the sample. The solid powder samples in MAS NMR probes are placed in rotors which vary in diameter from 2 to 7 mm, with smaller diameter rotors capable of higher spinning speeds for better averaging, while compromising signal due to the smaller sample size. These ceramic rotors are placed in a precision machined stator, which use gas bearings and gas driven impellers to achieve the high spin rates. In order to study material properties of solid samples at cryogenic temperatures, such as magnetic phase transitions, MAS probes have been developed which are capable of variable temperature (VT) experiments.
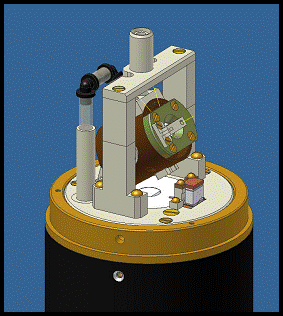
MAS Stator in 900 Probe
Current Cryogenic Delivery System The 900 MHz ultra-widebore NMR magnet at the NHMFL allows scientists to perform spectroscopy at higher sensitivities using large diameter probes, which can house more instrumentation than for similar narrow-bore 900 MHz magnets found in other labs globally. The RF Program of the NHMFL, led by Dr. William Brey and Mr. Peter Gorkov, have developed specialized NMR probes for this and other magnets at NHMFL which feature novel coil geometries, improving signal to noise ratio and reducing RF-induced sample heating in sensitive biological protein samples. The RF Program built a transmission line circuit MAS probe for the 900 MHz magnet, utilizing a Chemagnetics style MAS stator with a 3.2 mm diameter rotor. The probe was intended for use in VT experiments, and features a vacuum insulated glass dewar and internal probe heater for accurate delivery of cooled or heated gas to the sample chamber inside the stator.
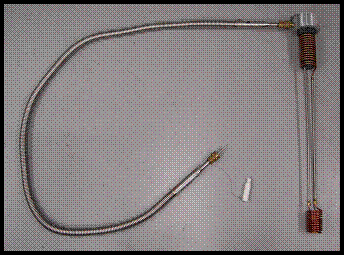
FTS refrigerator
For MAS experiments to temperatures reaching -70 °C, an Airjet XRII851 closed cycle refrigeration unit from FTS Systems is utilized to cool a room temperature nitrogen gas stream. This unit is capable maintaining a cold gas stream for volumetric flow of up to 1 standard cubic foot per minute (SCFM). When colder sample temperatures are required, the pre-cooled output of the FTS refrigerator is fed into a heat exchanger which is submerged in a liquid nitrogen bath inside a 50 L dewar manufactured by MVE Biological Systems. The heat exchanger was supplied by Doty Scientific, a manufacturer of commercial NMR and MRI probes and related accessories. It consists of counterflow pre-cooling coils, where incoming gas is cooled by the vaporized nitrogen at -195.8 °C which is exiting the dewar as it vents to atmospheric pressure. The gas then flows through the heat exchanger coils which are submerged in liquid nitrogen (LN2) and exits the unit through a stainless steel transfer line to the NMR probe. The transfer line is manufactured by Cryofab, Inc. and is vacuum insulated for higher thermal efficiency.
Doty Hx
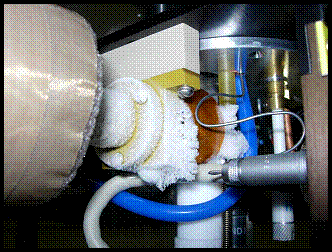
A design challenge with the current probe is where the metal transfer line adapts to the glass VT dewar at the base of the probe. Currently a Torlon adapter is used to mate with the brass quick-disconnect fitting at the exit of the transfer line, and then a small length of PTFE tubing is band clamped between the Torlon adapter and over the stem of the vacuum insulated glass dewar. This series of joints and adapters has proven to be an area where both gas leaks and higher thermal losses are evident from the formation of ice during cold bench tests of the system. Furthermore, since the transfer line enters the glass dewar at the horizontal but the length of the dewar is vertical, the Torlon adapter clamp must be secure enough to support the weight of the stainless steel transfer line, while at the same time allowing for thermal contraction in the vertical direction of the brittle glass dewar over the approximate 6 ft length of the NMR probe. Our client has explained that this is a desired area for improvement in our team’s final system design.
Ice Build-up
Also inserted into the base of the glass probe dewar is a resistance heater with internal thermocouple, used in conjunction with a PID temperature controller at the spectrometer console to provide final accurate setting of the sample temperature. The heater is manufactured by Bruker Biospin and is commonly used in their commercial NMR probes. The internal thermocouple provides a safety shutdown in the event of the heater temperature reaching an unacceptably high temperature either from electric fault or by removal of VT gas flow. There is a T-type thermocouple closer to the MAS stator which provides the actual feedback to the PID temperature controller. In a standard length NMR probe for smaller magnets, the distance between the heater and this VT thermocouple is approximately 2 to 3 feet, so there is not a significant lag between temperature detections and heater control. Since this probe is closer to 6 ft in length, there twice as much lag time in this feedback loop. It was expressed by our client that if our final system design requires the heater to be moved further upstream, such as somewhere in the transfer line, that sample temperature variations could possibly become wider and fall out of desirable limits.
The cooled gas stream travels up the probe through the glass dewar, which is actually a series of three interconnected glass dewars of shorter lengths to facilitate manufacture by the glass blower. The gas is routed through Tygon tubing in order to transition through a 180° U-turn since the VT entry port is located on the top of the MAS stator. Here, the gas blows on the sample rotor and surrounding solenoid, and exits the stator through an outlet port and out the top of the probe. It was determined during cold bench tests of the current system, that gas flows of 1 to 1.3 SCFM are required in order to cool the VT thermocouple to -188 °C. NMR experiments using temperature sensitive lead nitrate samples are planned for the near future to determine actual sample temperature under these conditions, since heating is introduced by the spinning of the rotor and from electric fields of the RF coil. |
