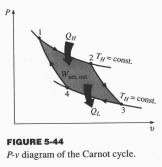
Carnot
Efficiency
Consider an ideal gas undergoing a Carnot cycle between
two temperatures TH and TL.
Ø 1 to 2, isothermal expansion, DU12 = 0
QH = Q12 = W12 = òPdV = mRTHln(V2/V1) (1)
Ø 2 to 3, adiabatic expansion, Q23 = 0
(TL/TH) = (V2/V3)k-1
(2)
Ø 3 to 4, isothermal compression, DU34 = 0
QL = Q34 = W34 = - mRTLln(V4/V3) (3)
Ø 4 to 1, adiabatic compression, Q41 = 0
(TL/TH) = (V1/V4)k-1
(4)
From (2) & (4): (V2/V3) = (V1/V4) Þ (V2/V1) = (V3/V4)
Since ln(V2/V1) = ln(V4/V3); substituting for ln(V4/V3) in (1)
Þ (QL/QH )= (TL/TH)
Hence: hth = 1-(QL/QH )= 1-(TL/TH)
It has been proven that hth = 1-(QL/QH )= 1-(TL/TH) for all Carnot engines since the Carnot efficiency is independent of the working
substance.
Example: A
typical steam power plant operates between TH=800 K (boiler) and TL=300 K(cooling
tower). For this plant, the maximum
achievable efficiency is 62.5%.