UNGRADED: One type of a multistep process is a cycle. Consider the
idealized car engine cycle, called Otto cycle. Assume that in state
1, the air-fuel mixture has been taken in and is still at 100 kPa
and 27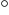 C ambient conditions. Then the air is compressed until
state 2, where the volume is one tenth of the intake volume. Assume
that this compression stroke is polytropic with
C ambient conditions. Then the air is compressed until
state 2, where the volume is one tenth of the intake volume. Assume
that this compression stroke is polytropic with 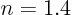 . Then from
state 2 to state 3, an isochoric 2,500
. Then from
state 2 to state 3, an isochoric 2,500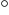 C increase in temperature
takes place. Finally, from state 3 to state 4, the power stroke,
the air expands polytropically with
C increase in temperature
takes place. Finally, from state 3 to state 4, the power stroke,
the air expands polytropically with 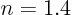 until the volume returns
to the original intake volume value. Finally, for the process from
4 back to 1, a true car engine would dump the hot air to the exhaust
and take in fresh cool air. However, the Otto cycle assumes that
the air at step 4 is isochorically cooled down from 4 to 1 to
the original intake temperature.
until the volume returns
to the original intake volume value. Finally, for the process from
4 back to 1, a true car engine would dump the hot air to the exhaust
and take in fresh cool air. However, the Otto cycle assumes that
the air at step 4 is isochorically cooled down from 4 to 1 to
the original intake temperature.
Model the air-fuel mixture as pure air. Find the pressure, specific
volume, and temperature at each of the 4 stages of the cycle. Then
find the net specific work produced in the complete cycle.
Also show the cycle and the work graphically in a very neat 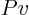 diagram.
diagram.
![]() diagram.
diagram.