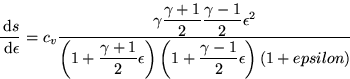- ...see
-
An interesting question is: how small is the entropy change across
weak shocks precisely? That can be seen from the full equation
(3'), written in terms of specific volume
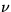 instead of density:
instead of density:
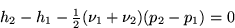
In the  plane, connect states 1 and 2 by a straight line:
plane, connect states 1 and 2 by a straight line:
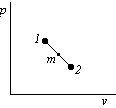
Note that the second term in the equation above is the trapezium rule
approximation for 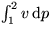 , and that the trapezium rule is
exact for a linear function. So along this straight connecting
line
, and that the trapezium rule is
exact for a linear function. So along this straight connecting
line
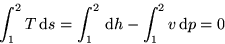
exactly. Now approximate the left integral with the midpoint rule:
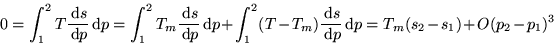
where m is the mid point between 1 and 2 in the 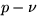 diagram.
It follows that the entropy change across a weak shock is very small,
proportional to the cube of the already small pressure difference.
diagram.
It follows that the entropy change across a weak shock is very small,
proportional to the cube of the already small pressure difference.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...grey.
-
The discussion above is a bit simplistic, since there are two
thermodynamic variables plus a velocity to approximate: in reality you
will need to approximate all three at the same time using shocks
moving left with the speed of sound, shocks moving right with the
speed of sound, and jumps that remain stationary compared to the gas.
The third set of jumps are called ``contact discontinuities'', rather
than shocks.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ...entropy
-
This is a pain. To do it, write (8a) and (8b) in terms of
the Mach number, M1*, write
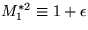 ,plug in the equation for the entropy,
,plug in the equation for the entropy,
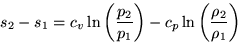
and take the derivative w.r.t. 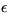 to get:
to get:
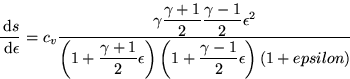
Which is always positive, so the entropy increase becomes positive for
 , i.e. M1*>1.
, i.e. M1*>1.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
![]()

![]()
![]()
![]()
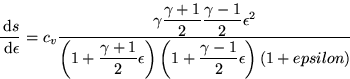
![]()

![]()
![]()
![]()