consider a volume ![]() let
let ![]() be the rate of energy got in
be the rate of energy got in ![]()
![]() is the rate of energy increased in
is the rate of energy increased in ![]()
![]() is the rate of work done by
is the rate of work done by ![]() on the surroundings.
on the surroundings. ![]() are from conduction +
radiation + heat source and sink.
are from conduction +
radiation + heat source and sink. ![]() is the rate of increase of internal energy and kinetic energy.
is the rate of increase of internal energy and kinetic energy. ![]() is the work done by potential forces and work done by stress. From the first law of thermodynamics (
conservation of energy)
is the work done by potential forces and work done by stress. From the first law of thermodynamics (
conservation of energy)
![]()
Work done by ![]() on the surrounding,
on the surrounding, ![]() can be written as
can be written as
![]()
Note :
![]()
![]()
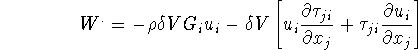
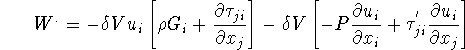
Energy increased in ![]()
![]()
e is the energy per unit mass.
![]()
![]()
Now
![]()
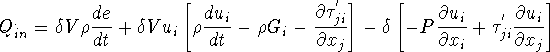
The first bracket is zero by Navier-Stokes equations thus,
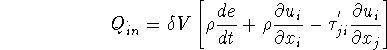
Rate of energy transferred into ![]()
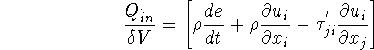
Net rate of heat conducted into ![]() in the x direction
in the x direction ![]()
![]()
Similarly for the y and z directions. Thus,
![]()
Then
![]()