
College of Engineering


|
Department of
Chemical Engineering
College of Engineering |

|
|---|---|---|
| Florida A&M University | Florida State University |
Experiment 300: Distillation
![]()
[ Theory] [ Manufactorers] [ Top] [ Back to UOL Home]
![]()
To follow
There are essentially two parts to the theoretical background regarding the analysis of the distillation column in our laboratory. The first area of interest is predicting values of the bottoms and distillate ethanol mole fractions based on the number of theoretical stages measured at total reflux and the known feed, bottoms, reflux, and distillate flow rates for the various experimental runs.
The second area of interest is determining the theoretical minimum number of stages and minimum reflux ratio based on the measured values of the bottoms and distillate ethanol mole fractions, not at total reflux.
The theoretical minimum number of stages is the number of stages needed to reach a desired separation. The theoretical number of stages at total reflux may be calculated using the Fenske Equation and the McCabe Thiele Model.
The Fenske equation may be used to calculate the theoretical minimum number of stages, Nm, at total reflux for a distillation column with a total condenser.
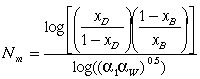
XD=mole fraction of desired product in distillate
XB= mole fraction of desired product in bottoms
a1 = relative volatility of the overhead vapor
aw= relative volatility of the bottoms liquid
The McCabe Thiele model may also be used to determine the number of theoretical stages at total reflux. The following steps will enable one to determine the number of stages:
Draw the equilibrium line on an x-y plot.
Draw the y=x line.
Plot the measured bottoms and distillate ethanol mole fractions along the y = x line. For total reflux this line will be your operating line.
Draw a horizontal line from the distillate mole fraction on the operating line to the equilibrium line, followed by a vertical line drawn back down to the operating line. Repeat this until the desired bottoms composition is reached.
Count the number of times the horizontal line hits the equilibrium line to get the number of stages. Note: The last stage is the reboiler.
The McCabe Thiele Model is used to determine the theoretical number of stages when not at total reflux.
Draw the equilibrium line on an x-y plot.
Draw the y = x line.
Plot the bottoms, feed, and distillate ethanol mole fractions along the y = x line.
Plot the q-line (feed line) starting from the mole fraction of the feed extending through the equilibrium curve. The q-line is the moles of liquid produced per mole of feed. The feed is pure liquid therefore the q-line is 1 and should be at a 90 degree angle from the operating line.
Plot the enriching operating line. The enriching operating line is calculated using the following equation:
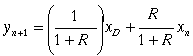
R is the reflux ratio.
xD= mole fraction of desired product in the distillate
xn is the composition leaving the nth stage.
yn+1 =vapor composition entering the bottom of the column
Plot the stripping operating line by extending a line from the bottoms mole fraction through the point where the enriching operating line intersects the q line.
Draw a horizontal line from the distillate mole fraction on the operating line to the equilibrium line, followed by a vertical line drawn back down to the operating line. Repeat this until the desired bottoms composition is reached.
Count the number of times the horizontal line hits the equilibrium line to determine the number of stages. Note: The last stage is the reboiler.
The reflux ratio R, relates the compositions of two streams passing each other. The following equation can be used to calculate the reflux ratio:
![]()
Ln is the reflux flow rate
D is the distillate flow rate
The minimum reflux ratio Rm is the reflux ratio that will require an infinite number of trays for a desired separation.
![]()
xD= mole fraction of desired component in distillate
y'=mole fraction in vapor where the operating lines touch the equilibrium line
x'= mole fraction in liquid where both operating lines touch the equilibrium line
Buffalo Technologies Corp.
www.buffalotechnologies.com
Tel.: 888-529-9925
Fax: 716-895-8263
Vendome Copper and Brass Works, Inc.
www.vendomecopper.com
Tel.: 502-587-1930
Fax: 502-589-0639
GEA Evaporation Technologies, LL
www.evaptec.com
Tel: 410-992-7400
Fax: 410-992-7426
Patterson Industries (Canada) Limited
www.pattersonindustries.com
Tel.: 800-336-1110, Ext.1078
Fax: 416-691-2768
Quark Enterprises, Inc.
www.quarkglass.com
Tel.: 800-462-7062
Fax: 800-462-7063
This section presents some pictures of the Distillation Column used for Experiment 300.
Front view of distillation column.
Control Panel.
Side view showing the column in relation to the control panel.
Back of the unit.
Bottoms Tank.
Close-up of the column showing the feed ports. (This column can be fed on different trays.)
Reboiler.
Varian Gas Chromatagraph and acompanyng Gateway Pentium 133 used for the data processing and printing of the GC's.
Please direct all inquiries to:
decoteau@eng.fsu.edu
![]()
[ Report Summaries] [ Data] [ Back Home]
![]()