When
a fuel is combusted, the energy it releases can go to three places: It can
produce work, as is done in a engine, it can manifest itself as heat transferred
to the combustion products (exhaust), or that heat energy can transfer through
the walls of the combustion chamber. †If
none of the energy released during combustion was to be lost through the walls
of the chamber or go to producing work, the energy transferred to the combustion
products would be maximized and the temperature of these products would be as high
as it could possibly be. †The
temperature of the products under this condition is called the adiabatic flame
temperature. The adiabatic flame temperature is never reached in practice, but
it is useful in design because it tells the engineer what the maximum exhaust
temperature of the exhaust could ever be and allows him/her to design accordingly.
Calculating the adiabatic flame temperature is an iterative process. †Since the temperature of the products is not known, guess values must be substituted into the energy equation.
An example
is useful to helping us understand the process. †In this case we will be using the same set of assumptions as we
did in the previous example. †The only difference
between that problem and this one is that we are assuming adiabatic flame
conditions
The chemical equation does not change it is still:
![]()
The† energy equation will change slightly this time around. Heat loss through the cylinder walls (Q) is being neglected:, so the enthalpy of the products is equal to the enthalpy of the reactants. †
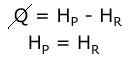
When the compounds and numbers of moles are substituted into the energy equation, the equation becomes:
![]()
Product
Reactant
The
reactant side of the equation can be easily handled. †The temperature of the reactants is already known and the enthalpy
values can be looked up in tables.
Letís take a closer look at the terms on the product side:
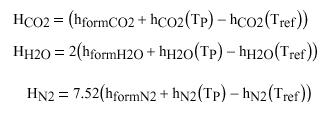
Tref and the enthalpy of formation are known, so those terms can be evaluated. †Tp is not known however. †This report is not about spoon feeding readers, so I will not go any further in showing you how to do adiabatic flame temperature problems. †I will only say that it is an iterative process.
Keeping it Real

Example of keeping it real
Earlier it was mentioned that the Adiabatic flame temperature is never reached in practice.† There are two main reasons why.† The first reason is that the high temperature of the exhaust products causes the compounds in the exhaust to dissociate, or break up. The heat energy of the products is used in breaking these bonds, so the temperature of the products is lowered. †Dissociation tends to decrease when the pressure at which combustion occurs is increased.† Secondly, no real combustion chamber is adiabatic. †Heat losses do occur through the wall of the chamber and this causes the actual temperature of the combustion products to fall further from the adiabatic flame temperature.