Thermodynamics |
||
| Introduction 1st law of thermo
2nd law of thermo
|
Thermodynamics: Science of Thermo (heat) and Dynamics (force or work). Physics that describes and correlates the physical properties of macroscopic systems of matter and energy and their capabilities to deliver work. Zeroth Law of Thermodynamics When two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium. As a consequence, all three bodies share a common property, that is the temperature. First Law of Thermodynamics The first law of thermodynamics is a law of energy conservation and it is based on experimental observations. It states that the amount of heat transferred into a system plus the amount of work done on the system must result in a corresponding increase of internal energy in the system. Second Law of Thermodynamics The second law of thermodynamics is sometimes called the law of entropy as it introduces the important property called entropy. Entropy can be thought of as a measure of how close a system is to equilibrium; it can also be thought of as a measure of the disorder in the system. The second law defines the direction which a specific thermal process can take place, and is sometimes given as a statement that precludes perpetual-motion machines of the second kind.
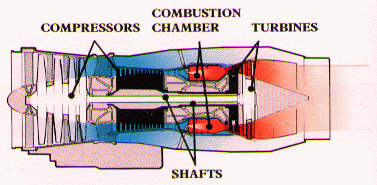
A Case Study: Jet Engines (from Rolls Royce Engine Company-it provides a qualitative understanding of how a jet engine produces thrust) A jet engine is used to provide the jet propulsion to power aircraft because they can produce very large power per unit engine weight. Air enters compressor and is being pressurized to a high pressure before entering the combustion chamber. Multistage compressor is usually used to achieve higher overall pressure. Compression ratios up to 30:1 can be achieved, resulting in extremely high efficiency and very low specific fuel consumption. Inside the combustion chamber, the fuel and air are mixed and ignited, which produces high temperature and high temperature gas expanding into the turbine section. The turbine consists of one or more stages of alternate stationary and rotating aerofoil-section blades. The turbine absorbs sufficient energy from the hot expanding gases leaving the combustor to keep the compressor rotating at its most efficient speed. See the following schematic to identify various components inside a jet engine.
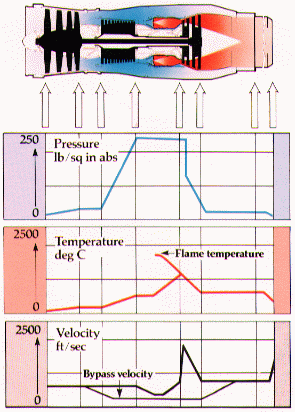
The following graphs show the changes in pressure, velocity and temperature of the gas as it passes through the various stages of a jet engine.
The thermodynamic process of a typical jet engine can be modeled using an ideal Brayton cycle, which consists of an isentropic compression, an isobaric combustion (heat addition) and an isentropic expansion processes.
Study Plan: (1) Thermodynamic Properties: Pressure, temperature and specific volume. p-v-T relationship, phase change, property tables, idea gas equation and other equations of state. (2) First law of thermodynamics: Heat, work and internal energy change. Closed and open system analysis, steady state flow processes. (3) Second law of thermodynamics: Carnot cycle, reversible and irreversible processes, thermal efficiency. (4) Entropy: What is entropy? Isentropic processes, irreversibility. (5) Thermodynamic Cycle Analysis: Vapor power cycles (ex. Rankine cycle), Gas power cycles (ex. Diesel and Stirling Cycles), Refrigeration cycles (ex. refrigerators and heat pumps). |
|