
Design of a Bone Density Indenter
Team 102: Sponsored by Exactech
Exactech, a manufacturer of replacement shoulder joints, wants to create a tool to measure bone quality quantitatively. Exactech asked the FAMU-FSU College of Engineering to make such a device. Bone quality is an important factor in shoulder replacement surgery.
Age, injury, disease, or a combination of these, can cause damage to the shoulder joint. When a joint is damaged, shoulder replacement surgery is a treatment option. The surgery removes the damaged joint, replacing it with an artificial joint. These artificial joints fall into two general categories, stemmed and stemless implants. Stemless implants provide shorter recovery times and less invasive surgeries. However, these need a sufficient humeral bone quality. If the bone quality is not acceptable for a stemless implant, the surgeon uses a stemmed implant.
To determine the quality of the bone the surgeon uses a “Thumb Test.” The humeral head is cut off, then the surgeon places their thumb on the cut plane of the bone. The surgeon then uses their thumb to apply pressure to the bone. Based on the bone’s deflection, the surgeon discovers the bone quality and implant type. However, this is a qualitative measurement based only on the surgeon’s experience.
The team designed a tool that replaces the subjective “Thumb Test” with a handheld indenter which creates a quantitative score of bone quality. The indenter uses a spring to accelerate an indenting pin. This causes the pin to strike the cut face of the bone. The tool measures the maximum distance the pin penetrates the bone. The distance the indenter traveled identifies the bone quality. The pin enters the portion of the bone that is removed as part of the surgery, which prevents interference between the measurement and the replacement joint.
Initial Concept
This was the original design to replace the thumb test. The device sits between the cut face of the bone and the surgeon's thumb. This allowed the surgeons to provide the force applied to the bone. As force was applied the spring would compress and display the force it experienced on the side.
First Prototype
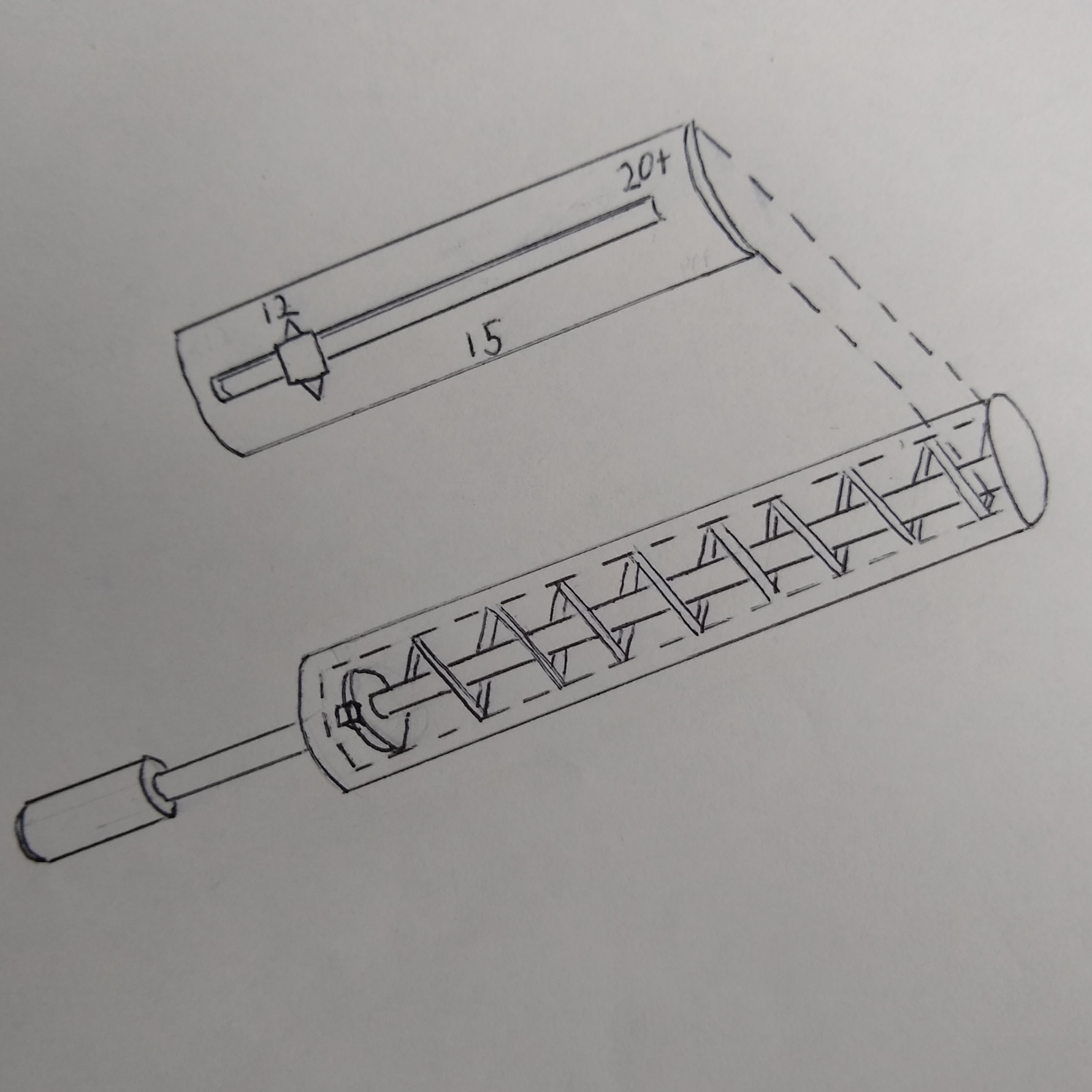
The device was revised to use a spring as the source of force rather than a surgeon's thumb. This ensures that the indenter will apply the same force in every measurement. This removes the surgeon as a source of potential inconsistency. The indenting tip is also constrained by the housing, limiting the depth the indenter can penetrate the bone. The device operates by pulling the handle back compressing the spring. The operator then turns the handle to catch the indenter guide on a shelf milled into the housing. The device is placed against the bone and the surgeon turns the handles to release the indenter from the locked position.
Current Prototype

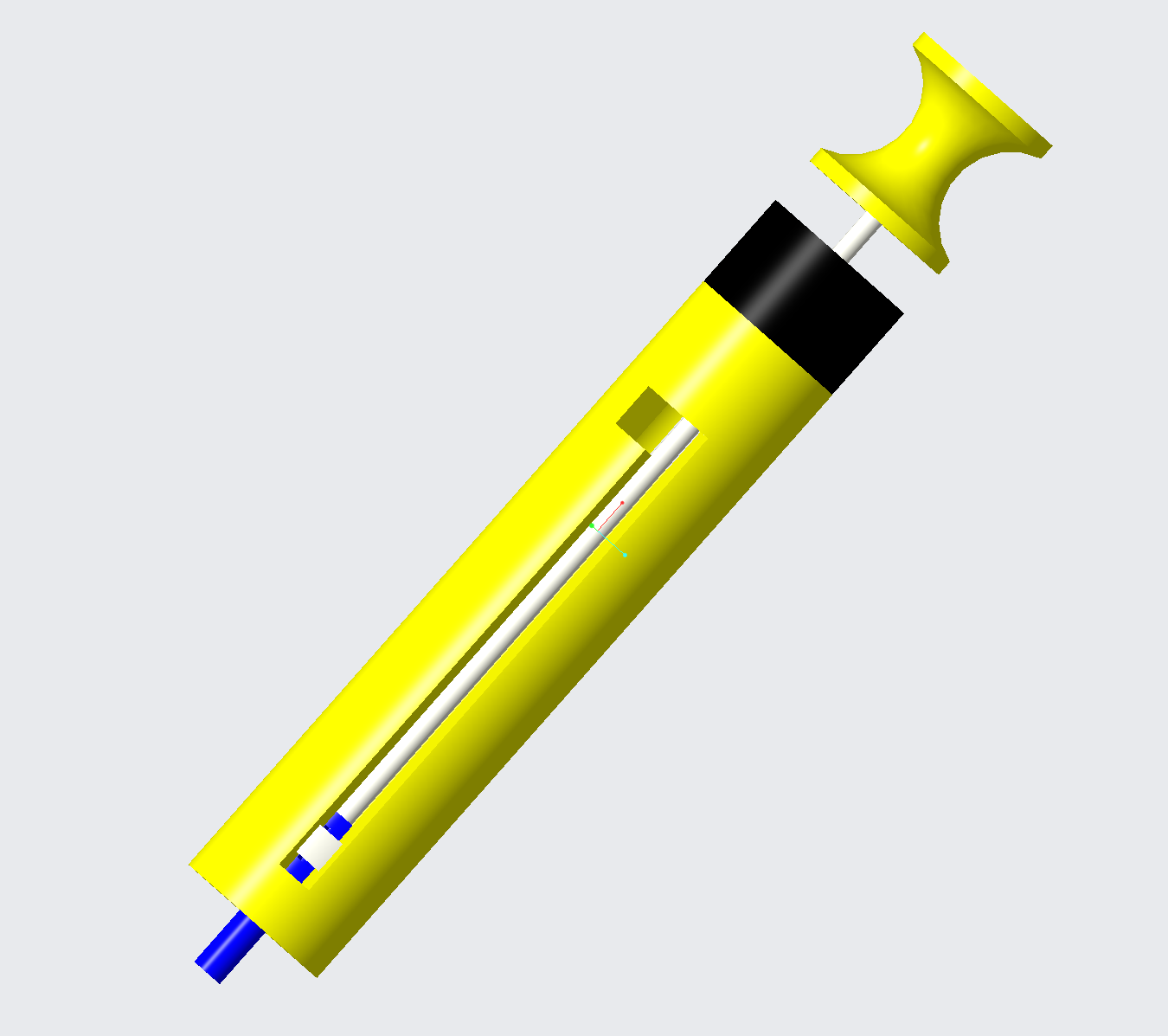
The design was refined to use a button release. The indenter tip is now a interchangeable part, seperate from the indenting rod, simplifying the manufacturing. To operate the device the surgeon pulls the handle back until the device locks. The surgeon then holds the device against the bone and presses the button. This process can be done with one hand, a noticeable improvement over previous models.
Fig. 1: Test Set-up
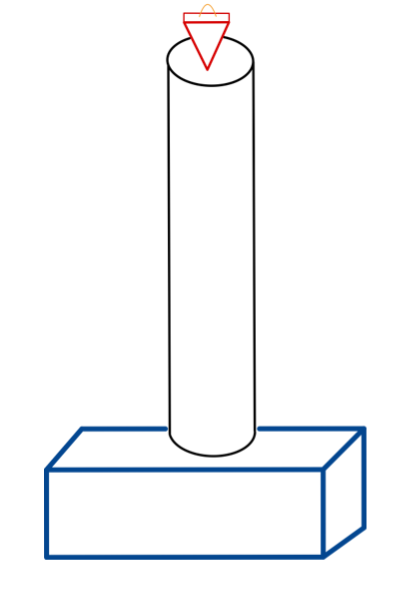
To determine the stregth of spring required for the device to indent bone, drop testing was done using the saw bones sent to the team by Exactech. The test set up is shown above in Fig. 1. The blue box represents the sawbone block. The cyclinder represents a 50 in. pipe. And the red pyramid represents a weight with a pyramid shape. The cyclinder was placed on top of the sawbone and weights of different sizes were dropped through it. The cylinders purpose was to ensure the weight would fall the same distance and the in the same orientation each trial.
Fig. 2: Initial Drop Testing
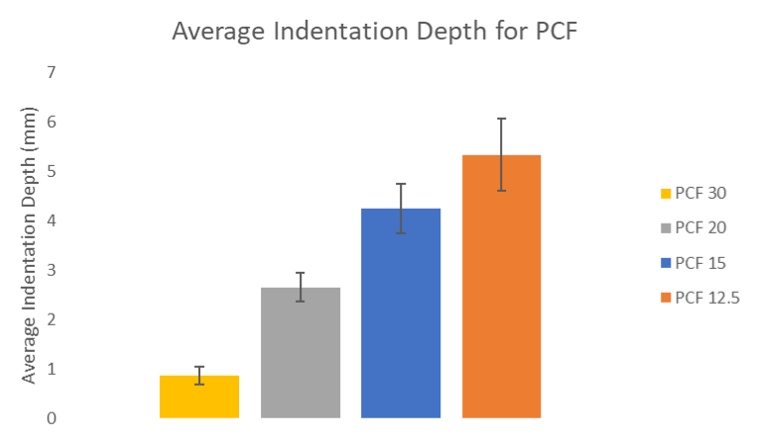
During the testing the data was recorded. The team took note of the mass of each weight, the height from which it was dropped and the indentation depth each collision created. For the first analysis the same weight and height was used in each trial. The graph in Fig. 2 above compares the indentation depth of 3 samples with diffferent densities measured in pounds per cubic foot or PCF. The results showed a linear relationship between PCF and indentation depth.
Fig. 3: Flatpoint Drop Tesing
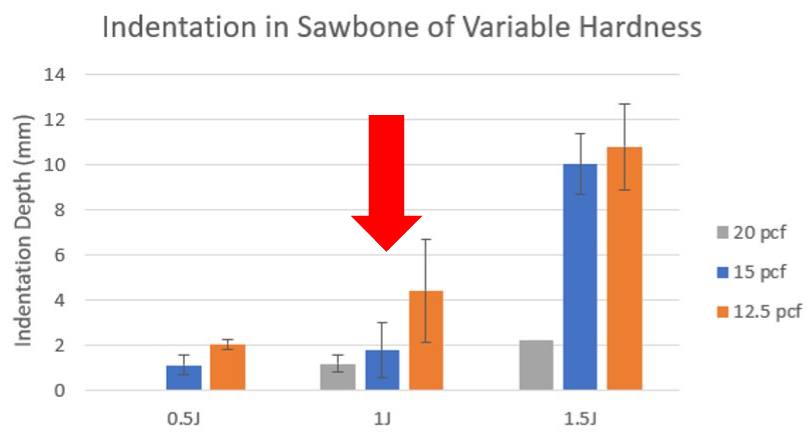
The next phase of testing was done with a 3D printed flatpoint indenter tip with a diameter of 0.1 in. instead of the weights used previously. The same test set-up was used, and the height was kept constant but the weight used was varied, with the purp[ose of indenting each block with varied potential energies. Each of the blocks was indented with 0.5J, 1.0J and 1.5J of potential energy. The results showed the largest difference in indentation depth when the samples were exposed to 1J of potential energy. This information was then used to choose the device's spring coefficient and compression.
The indenter’s physical properties and performance were compared to the targets set at the beginning of the project. The device’s physical dimensions and weight were within the targets set. More importantly, the device was sterilized and used to indent sawbone samples. The device survived the autoclaving sterilization process, before accurately indenting the sawbone samples. The process was repeated until the device experienced mechanical failure, at nearly twice the intended number of uses. The results of the testing showed all targets were met or exceeded.
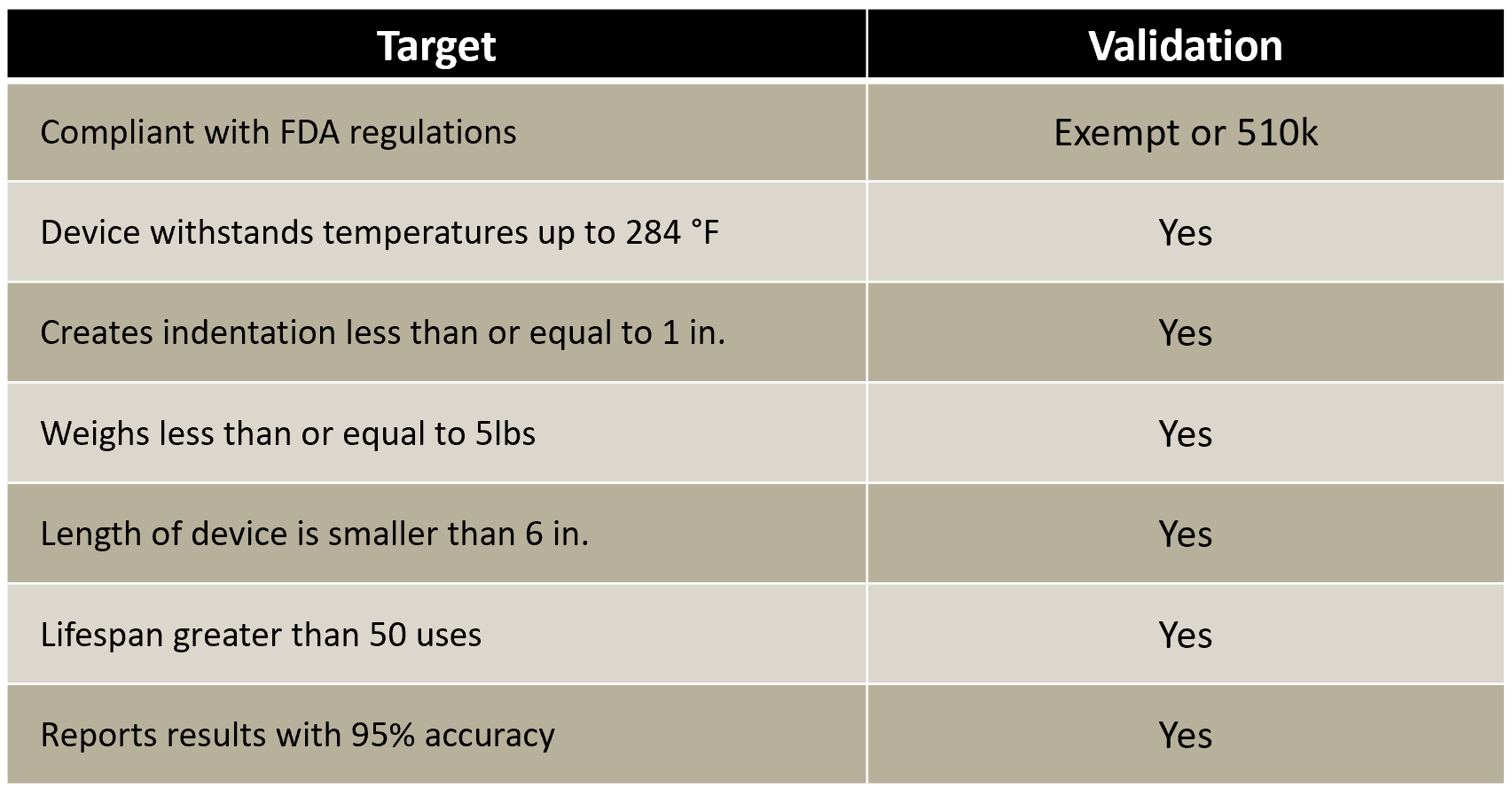
Based on the results of validation testing and discussions with our sponsor, further improvements to the design were identified. These changes include the use of hardened stainless steel for the indenter tip to improve device life. Additionally, welding the indenter tip will improve the accuracy of the device’s measurements. Further changes are proposed to simplify the assembly and sterilization process to convert the prototype created into a final design for production.
Orthopedic Bioengineer
gpg18d@my.fsu.edu

Grant is a senior in the BME department originally from Valparaiso, IN. He will pursue a master’s degree in the field before joining the industry workforce with plans to study in a JD-MBA program in his free time later in life. He has taken a particular interest in orthopedics and prosthetics and wants to help create a world where pain free life is achievable for everyone
Clinical Engineer
amg18e@my.fsu.edu

Abrea is pursuing a Bachelor of Science Biomaterials and Polymers Engineering at Florida State University. She is born and raised in Lafayette, AL. Abrea is involved in the W.E.B DuBois Honor Society and Biomedical Engineering Society. She upon graduation she hopes to pursuing a career in clinical engineering. Abrea hopes to go back to school to work towards her Ph.D.
Biomaterials and Biopolymers Engineer
eap18@my.fsu.edu

Erin is a Biomaterials and Biopolymers major at Florida State University. She grew up in Daytona Beach, Florida, and moved to Tallahassee to pursue Biomedical Engineering. She currently works with Dr. Locke and Dr. Rodriguez to design a Coupled-Plasma Bioreactor to break down pollutants as an alternative to harmful chemicals. Erin plans to continue her education by pursing a PhD at Florida State.
Materials Engineer
tas18d@my.fsu.edu

Tessany is a Mechanical Engineering student, who is also pursuing a minor in Computer Science. She was born in New Hampshire, but early on moved to Miami, FL where she graduated high school. Tessany is very involved in the FAMU-FSU Theta Tau Professional Engineering Fraternity. Tessany hopes to have a career in the medical device industry and go back to school for higher education after gaining some experience.
Manufacturing Engineer
tjs11f@my.fsu.edu

Timothy is from Ft. Lonesome, Florida and is a senior in Mechanical Engineering. He is interested in design for manufacturing, and additive manufacturing. He enjoys applying what he has learned in classes to the real-world problems. To this end he has worked in a 3d print farm, manufacturing facility, and a clay mine. When not working he enjoys collecting degrees; this will be his third.
Bioinstrumentation Engineer
njv18b@my.fsu.edu

Nick is a senior in the Biomedical Engineering program at Florida State. He will be pursuing a M.S. in Biomedical Engineering through the BS-MS program here at the FAMU-FSU College of Engineering. After earning a Masters degree, Nick will enter the industry in hopes of creating medical instrumentation and devices that can greatly benefit in people's lives.
The deliverables below show the current progress of our project.
2525 Pottsdamer St, Tallahassee, FL 32310
