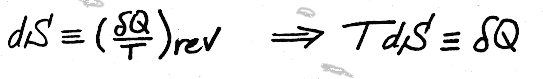 ,
,
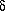 Q = dU +
Q = dU + 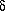 W
W
and
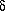 W = pdV
W = pdV| previous | page 1 | page 2 | page 3 | page 4 | page 5 | page 6 | next |
|---|
For saturated liquids, saturated vapors and superheated vapors, we are able to calculate an entropy change by looking up the entropy for each state in the thermodynamic tables. But it is not so straight-forward for solids liquids and ideal gases...
Remember,
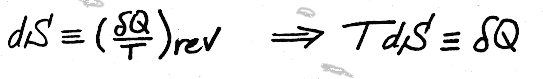 ,
,
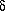 Q = dU +
Q = dU + 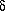 W
W
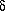 W = pdV
W = pdV
Now, remembering that H = U + pV,
| previous | page 1 | page 2 | page 3 | page 4 | page 5 | page 6 | next |
|---|