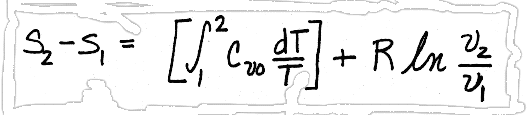 and
and 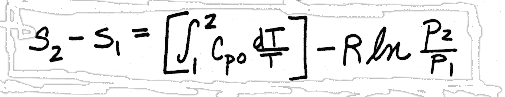
| previous | page 1 | page 2 | page 3 | page 4 | page 5 | page 6 | next |
|---|
Now we will approach the solutions to
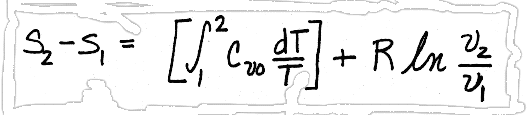 and
and 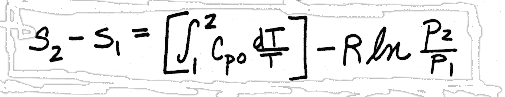
I. The least accurate method would be to assume that Cvo and Cpo are constant with temperature, and pulling them through their respective integrals, giving
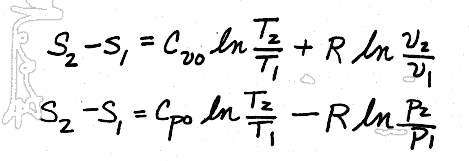
II. A more accurate method would be to get equations for Cvo and Cpo as functions of temperature, and integrate using those equations, giving
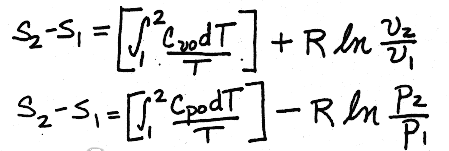
III. The most accurate method for determining the entropy change for ideal gases is to used the values for entropy at each of the given temperatures, and be sure to make an adjustment for pressure, and calculate the change exactly, as shown in
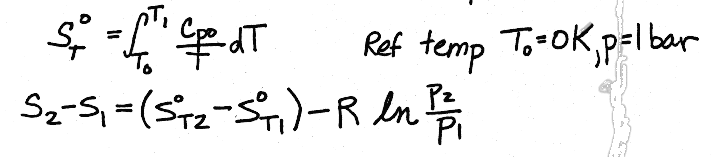
You should now be able to complete homework #17
Have a great holiday, and I'll see you on July 10th.
-Dr. P.H.
| previous | page 1 | page 2 | page 3 | page 4 | page 5 | page 6 | next |
|---|