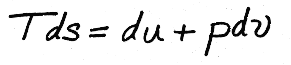
| previous | page 1 | page 2 | page 3 | page 4 | page 5 | page 6 | next |
|---|
The solution for ideal gases is not as simple as that for solids and liquids because
For IDEAL GASES we can say that
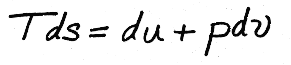
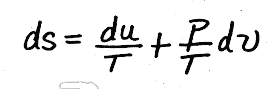
Before in our treatment of internal energy for ideal gases, we determined that
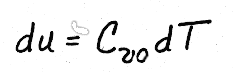
 .
.Substituting for du and p/T, we get,
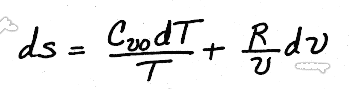
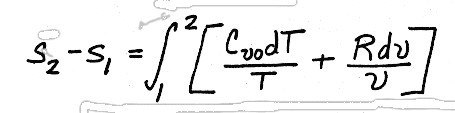
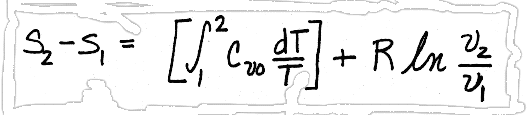
We will look at how to use this solution later, but for now, we will consider how to use enthalpy to determine entropy changes in ideal gases.
| previous | page 1 | page 2 | page 3 | page 4 | page 5 | page 6 | next |
|---|