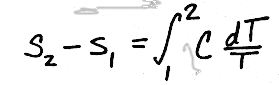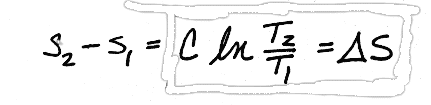EML3100 Thermo - Summer 2001 - Web Lesson
Entropy changes in solids liquids and ideal gases - PAGE 3

For SOLIDS and LIQUIDS we can say that
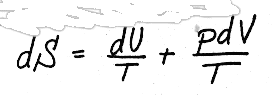
or, if we divide through by mass to get the intensive properties,
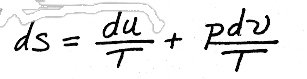 .
.
Solids and liquids are fairly incompressible, meaning dv ~ 0, leaving
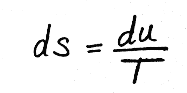 .
.
Before in our treatment of internal energy for solids and liquids, we determined that
du = CdT,
where, C was the specific heat for the solid or liquid, and would be treated as constant with temperature for our problems. Substituting this in the above equation gives,
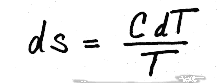 .
.
Now, to get the entropy change we must integrate, giving
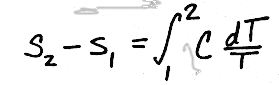
and since C is assumed to be constant with temperature, we can pull it through the integral, giving us
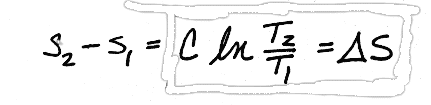
Values for C can be found in table A-19.
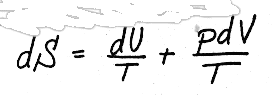
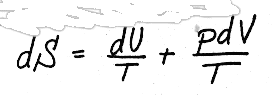
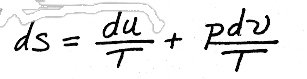 .
.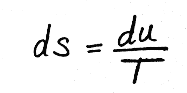 .
.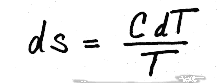 .
.